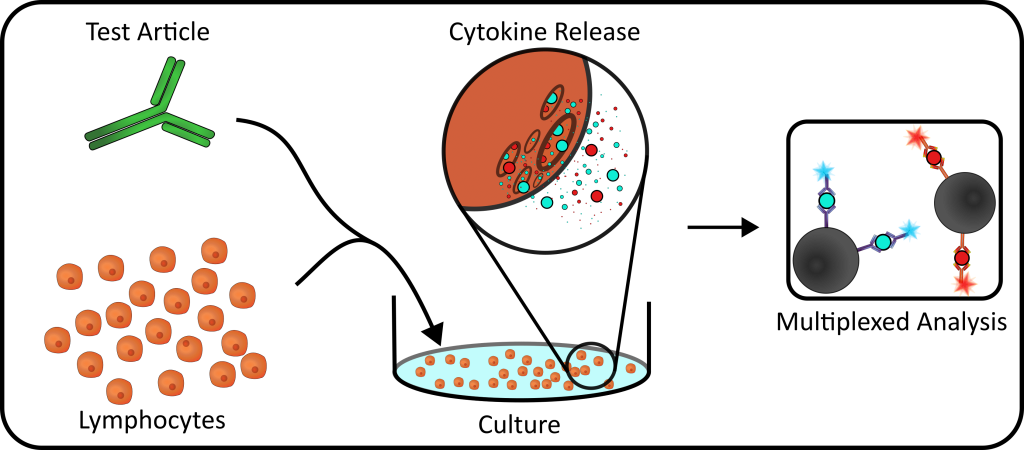
Background: Leukocytes express a variety of cytokines and chemokines in response to antigenic stimulation. Both the type of cytokine expressed, pro- or anti- inflammatory, and the relative concentrations predict the overall cellular response to a stimulatory event. Due to systemic organ damage that ensues with excessive quantities of pro-inflammatory cytokines (cytokine storm), testing of immune therapeutics with the potential to activate lymphocyte cytokine production is an important patient safety measure.
Principle: The basic cytokine release assay is performed by culturing the test article with either whole blood (WB) or blood derived lymphocytes (PBMC) and the culture media collected and tested for expressed cytokines. Cytokines are measured by multiplex bead technology using MILLIPLEX® MAP based on the Luminex® xMAP technology allowing for analysis of multiple cytokines simultaneously.
References:
Related Assays:
"*" indicates required fields
Copyright © 2021. All rights reserved.