The field of immuno-oncology is as vast as it is complex. From understanding the effects of the tumor microenvironment on innate immune cells, to checkpoint inhibitors for T-cell activation, there is no doubt that developing a novel therapy requires significant time, investment, expertise, and attention to detail. Xeno Diagnostics’ small group of dedicated and knowledgeable scientists has decades of expertise in in-vitro assays for early-stage drug development. We support biotech companies, pharmaceutical and academic life science organizations to enhance the quality and speed of their research. Our suite of custom assays and personal interaction with investigators have been designed to move the process forward. We are truly dedicated to making a difference for our clients.
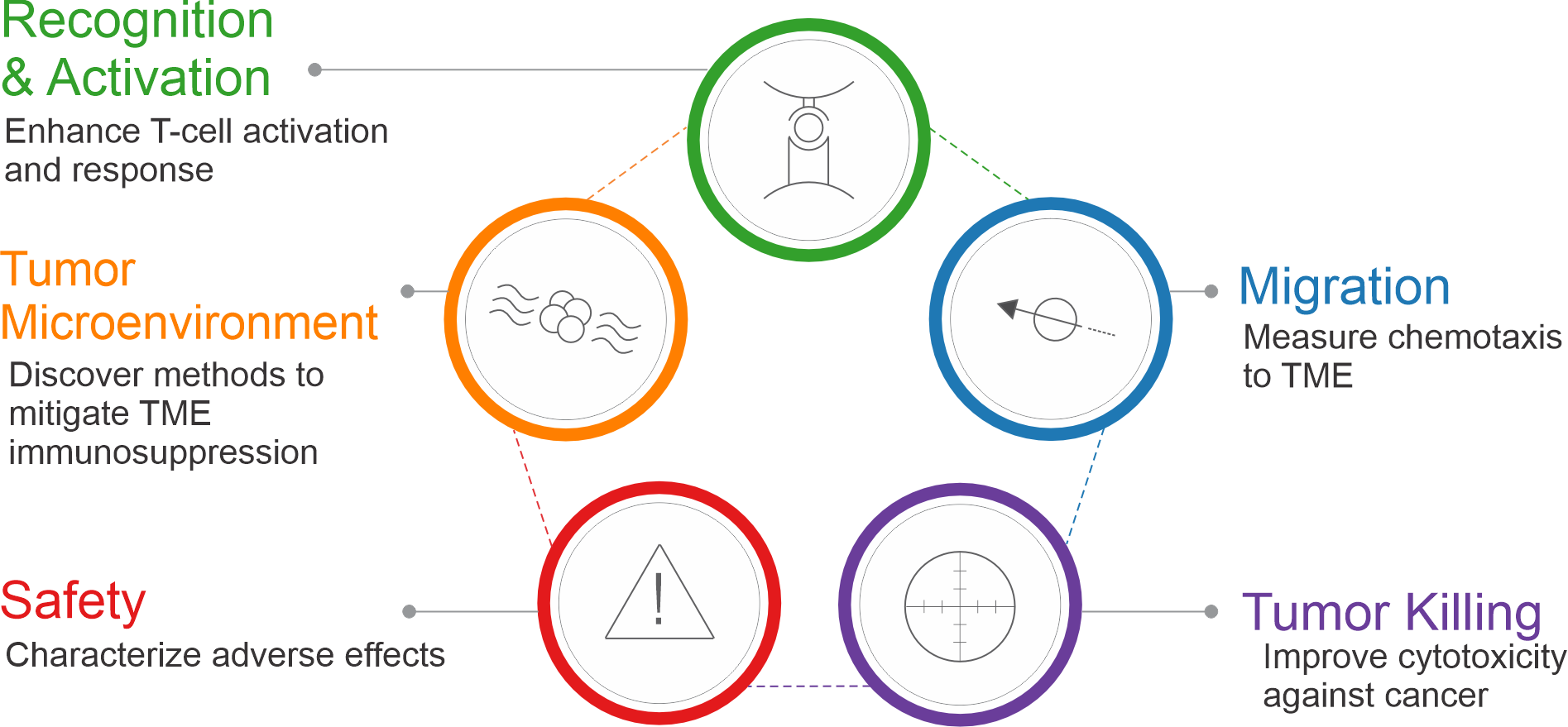
Xeno Diagnostics offers a suite of assays tailored to address the numerous mechanisms and modes of cancer development embodied by today’s novel therapies. While there are many different areas of research in immuno-oncology based on the type of cancer and the mechanism of the therapy, Xeno Diagnostics broadly categorizes these into 5 groups or phases: Tumor Microenvironment effects, tumor and/or antigen Recognition and Activation of immune cells, recruitment of immune cells and Migration towards the tumor, Killing of the tumor by cytotoxic cells, and evaluating the therapy for Safety and adverse effects.
Xeno Diagnostics is a CLIA certified high complexity lab and adheres to GLP and GCLP standards. We have had years of experience helping clients navigate the regulatory landscape and produce high-quality assays, documentation, and reporting to fit the regulatory requirements for each stage of product development.
Copyright © 2021. All rights reserved.