The Mixed Lymphocyte Reaction assay (MLR) is a highly customizable assay used to illuminate many activities of cell-based systems. MLRs are one of the primary in-vitro assay formats to investigate the immunomodulatory potential of new drug candidates or to gauge the relative immunogenicity of novel cellular therapies.
The MLR can also be used for screening in discovery / preclinical stages as it provides valuable information about the efficacy and mechanism of action of your product. MLRs are an effective in-vitro model to assess lymphocyte activation, inhibition, or alteration of function. The customizability of the assay also allows us to investigate interactions and responses of specific lymphocyte subsets and better identify which cells and under what conditions your product is most active.
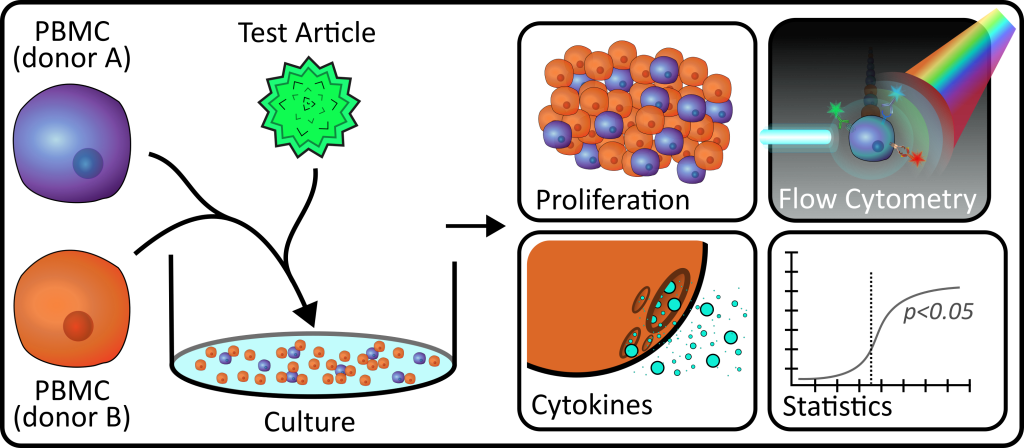
Mixed Lymphocyte Reactions (MLRs) are a great laboratory assay to help us understand how newly developed medicines interact with our immune cells and can provide researchers valuable information on the mechanism of action, efficacy, and safety of their product.
At its simplest, MLRs are comprised of at least two cellular components (lymphocytes) mixed together in a co-culture. In a one-way MLR, one group of lymphocytes act as a stimulator and another group of lymphocytes as a responder. The stimulator lymphocytes are often treated with a drug (or irradiated) to prevent them from proliferating or from mounting an immune response. This means that when we analyze the MLR, the only lymphocyte activity will be from the responder group. The other type of MLR is a two-way MLR, where both groups of lymphocytes act as both responders and stimulators against each-other.
Two-way MLRs are a good way to get a basic understanding of how a therapeutic product affects lymphocytes, while a one-way MLR allows us to dive into greater detail on the effects in one lymphocyte group and account for differences between the stimulator and responder lymphocytes groups (such as allogeneic donor variation if cells are from two different human donors).
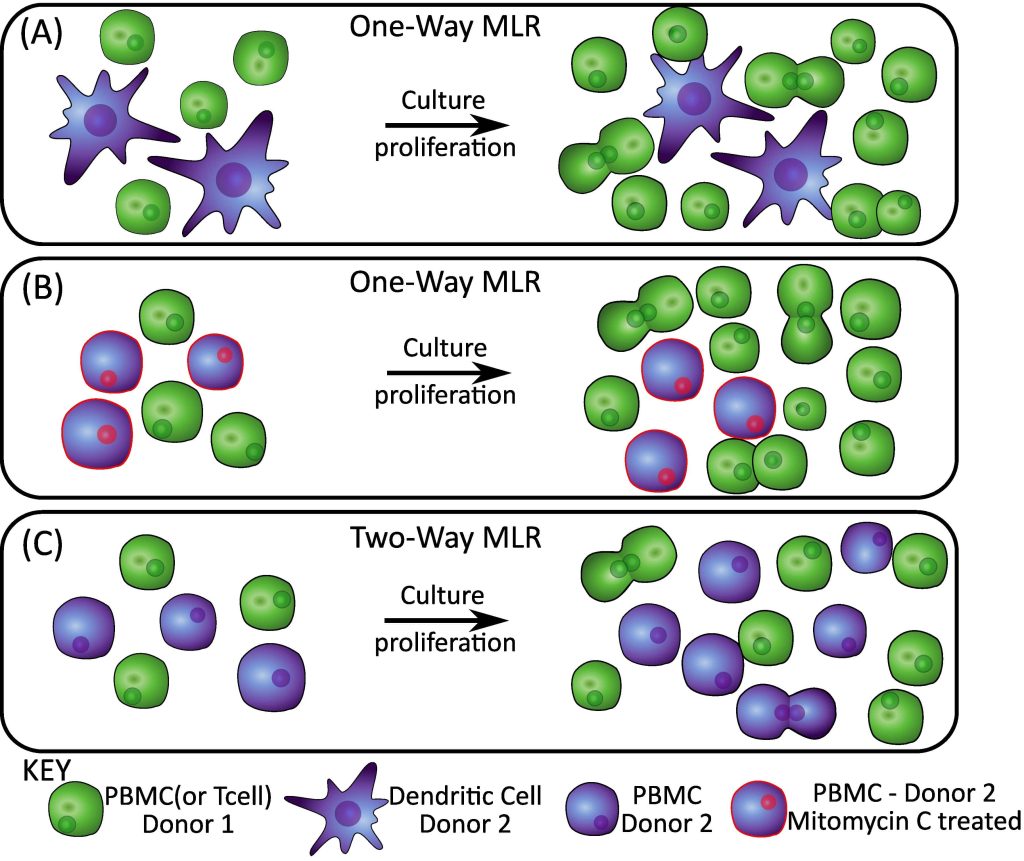
Another variation of the MLR, the DC/T-cell model, is useful for probing the initiation of an immune response and investigating how a therapy affects this response. Dendritic cells are Antigen Presenting Cells (APCs) which present foreign antigens (small molecules or proteins that are derived from the foreign invader that our body is trying to fight) to T lymphocytes (T-cells). Once a DC cell presents the antigen to the T-cell, the T-cell becomes activated, proliferates, and starts acting to help clear the infection.
Readouts from the MLR are selected depending on the mechanism of interest. See Figure 2 for some of the most common readouts. Xeno Diagnostics has a suite of readout options tailored to common mechanisms of interests. We work with our clients to design experiments with these readouts in mind and we assist with data analysis and interpretation on the backend.
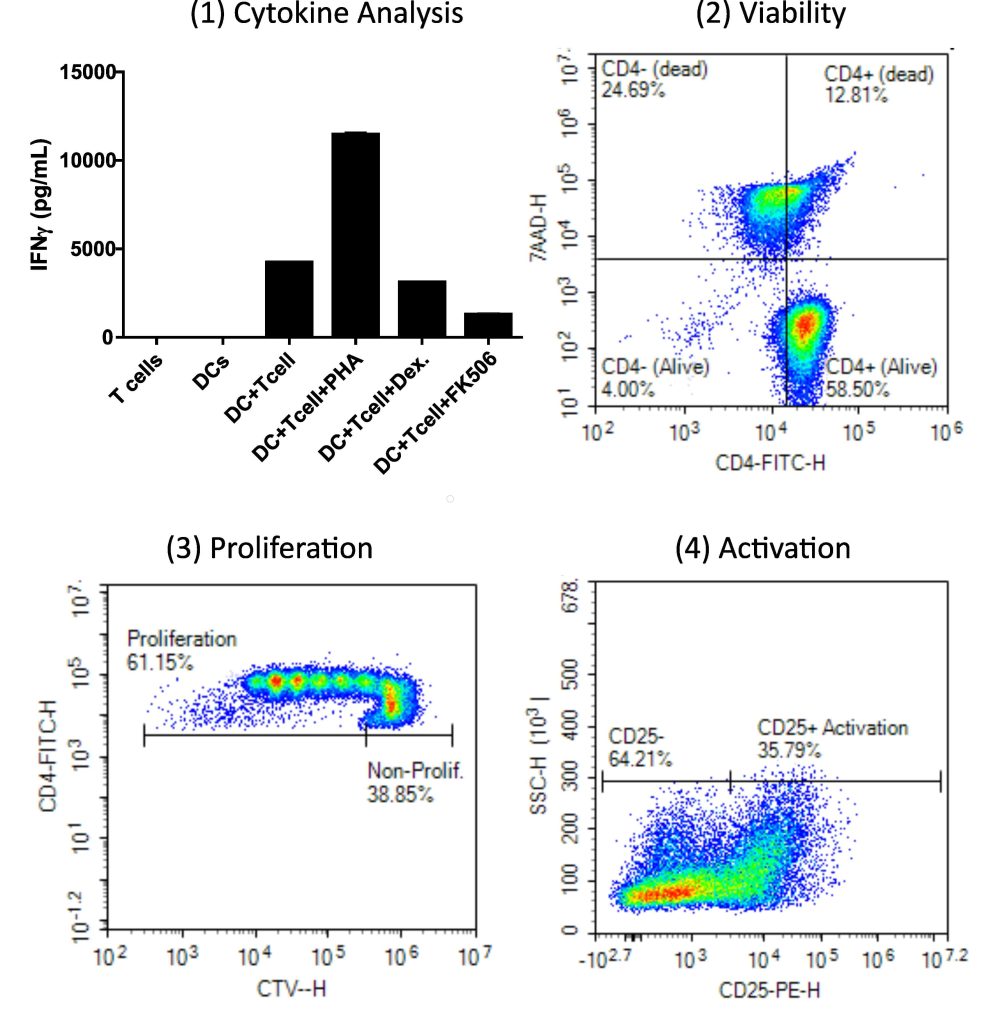
Figure 2 – Read-outs for an MLR. 1) IFNy activation/stimulation of test populations with positive and negative controls. 2) Viability of test population with flow cytometry. 3) CTV staining for cell proliferation with flow cytometry. 4) Identification of CD25 surface markers on test populations to identify T cell activation.
This experiment characterized the effects of co-culturing different ratios of dendritic cells (DCs), loaded with tetanus toxoid (TT), with purified T lymphocytes (1:10, 1:20, 1:40, 1:80, and 1:160) on T cell proliferation and cytokine production.
Pan-T cells were isolated by negative immunomagnetic separation from human peripheral blood mononuclear cells (PBMCs) and were placed in serum free AIMV media. Human monocytes isolated from PBMCs were cultured and matured for 24 hours in media containing IL-4 and GM-CSF (in 10% FBS/RPMI) followed by the addition of tetanus toxoid (5 µg/mL) for an additional 24 hours. At 48 hours of culture, media containing PGE2/TNFα/IL-6/IL-1β was added to the maturing macrophage/dendritic cells. At 72 hours of culture, media was removed and replaced with previously isolated T-cells in AIMV media containing sCD40L and the cells co-cultured for an additional 3 days.
At the end of the culture period, media supernatants were analyzed for cytokines (IFN-γ, IL-4, IL-6, MIP-1α, TNF-α, IL-10 and IL-12) via Luminex MagPix™ bead technology. Co-cultured cells were analyzed for cytokine analysis utilizing BrdU incorporation and ELISA on Day 6 (Figure 3).
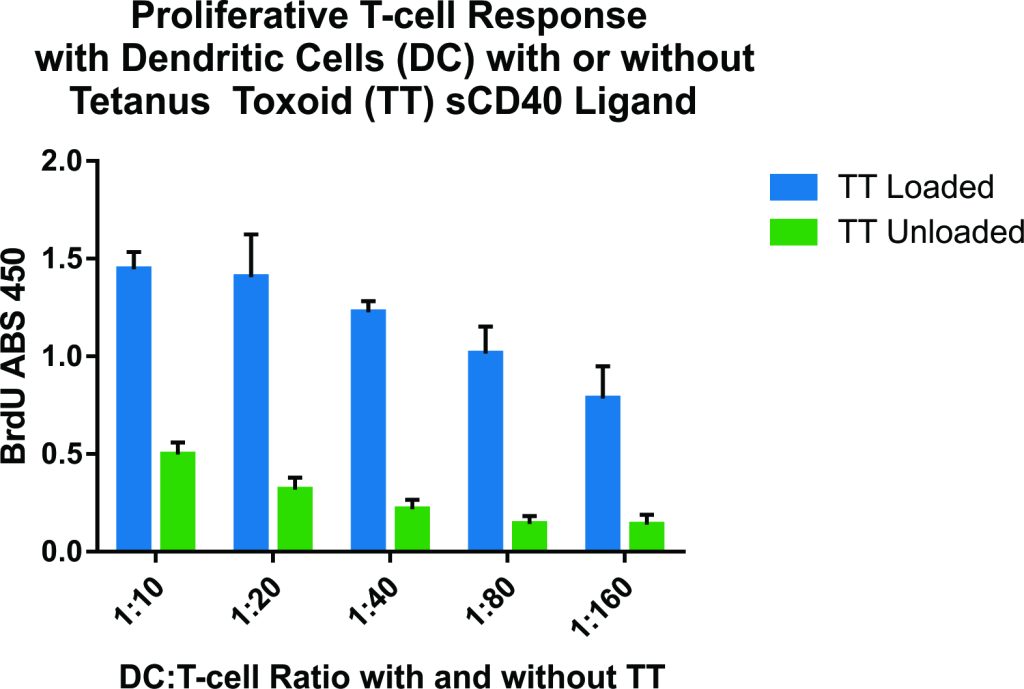
Figure 3 – Optimal stimulation by the TT-loaded dendritic cells was observed at DC/T cell ratios of 1:10 and 1:20.
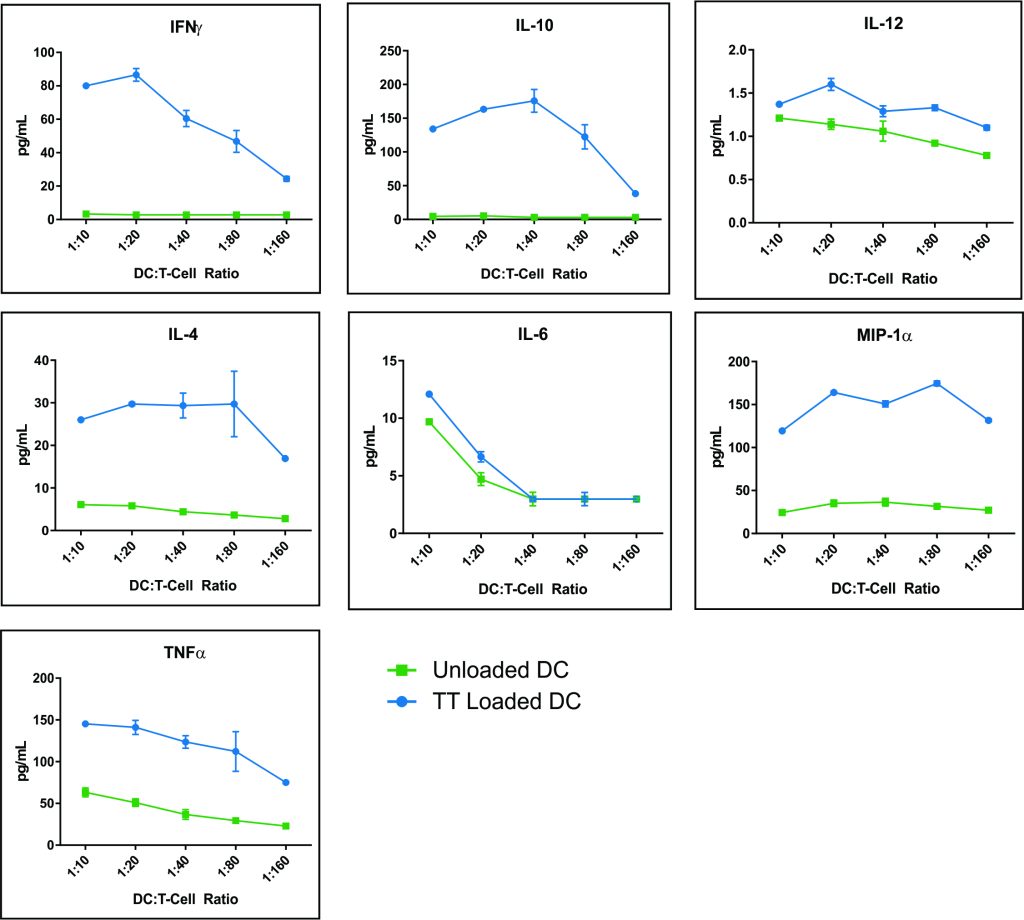
Figure 4 – Co-cultures at 1:10 and 1:20 DC/T ratios had increases in each of the 7 cytokines analyzed (IFN-γ, IL-4, IL-6, MIP-1α, TNF-α, IL-10 and IL-12). At higher DC/T cell ratios there was a general tendency for a reduction in cytokine release, especially for IL-6 and IL-12 (Table 1). The relative order of magnitude of cytokine secretion was IL-10> MIP-1α>TNF-α>IFN-γ>IL-4>IL-6.
Immunogenic response (HLA mismatch/allorecognition) was observed for co-culturing PBMCs from two different human donors in a 7-day one-way allogeneic MLR.
Five different responder human donor PBMC samples were co-cultured with a single human PBMC stimulator in a 7-day one-way allogeneic MLR. The stimulator cells were treated with a DNA crosslinker to arrest proliferation of the stimulator PBMCs before being added to the co-culture. At the end of the culture period, media supernatants were analyzed for TNF-α via Luminex MagPix™ bead technology.
Due to the growth arresting treatment, any proliferation observed is due to the allogeneic response of the five responder PBMCs. Figure 5 below shows the variability of TNF-α responses in the donors (A, B, C, D, and E) subjected to the same individual stimulator (F). Depending on the exact human leukocyte antigens (HLA) that each donor PBMC expresses, and the individual tolerances of each donor, allogenic responses will vary for each donor pair tested. In the above example, Donor #E has the greatest allogeneic response as measured by the inflammatory cytokine TNF-a when cultured with non-HLA matching donor #F. All allogeneic conditions show greater response than baseline (background) single donor PBMC controls for each individual donor.
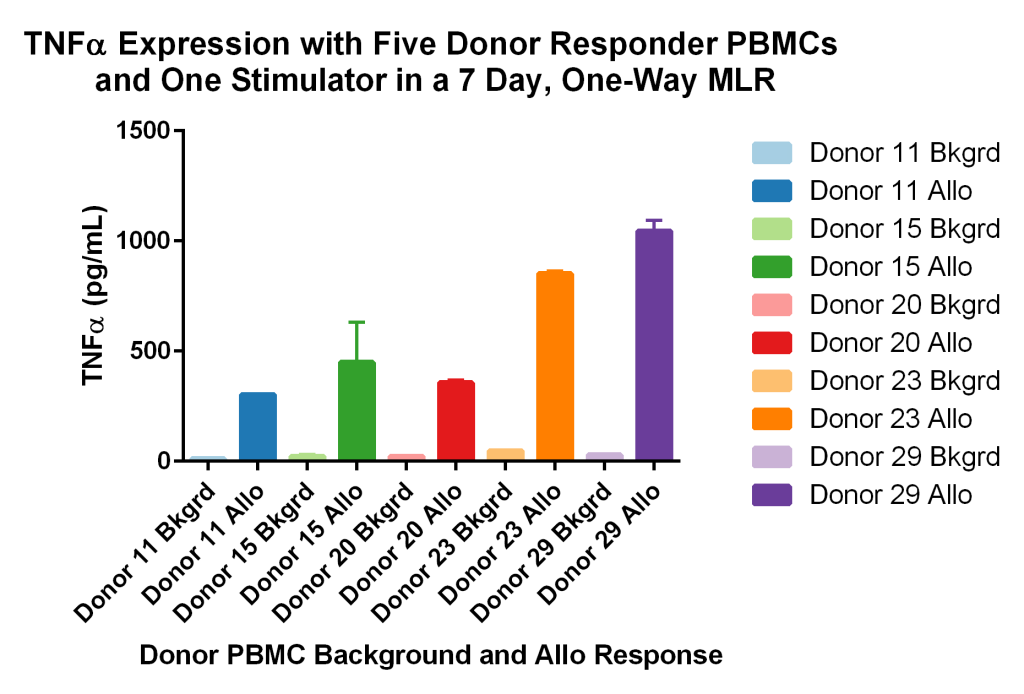
Figure 5 – Immunogenic responses (HLA mismatch/allorecognition), as determined by TNF-alpha secretion, were observed upon co-culturing PBMCs from different human donor pairs in a 7-day one-way allogeneic MLR.
Xeno Diagnostics offers a comprehensive selection of MLRs and co-culture experiments to test your product. We have designed MLRs for a wide range of applications including immuno-oncology, transplant immunology, and regenerative medicine. We can help you fine-tune your cell-based experiment to pin-point the system and processes you are looking to characterize.
We offer both basic MLR assays to initially screen your product, and fully customized MLR assay to fit your specific needs. We specialize in custom assays and work hand-in-hand with our clients during experimental design to ensure that data generated is pertinent to their exploratory question or regulatory requirement.
"*" indicates required fields
Copyright © 2021. All rights reserved.