Xeno Diagnostics is an accredited, high complexity testing laboratory under the Clinical Laboratory Improvement Act (CLIA) and is also compliant with all Good Laboratory Practices (GLP) and Good Clinical Laboratory Practices (GCLP) requirements. The Good Clinical Laboratory Practices (GCLP) concept embraces both the research and the clinical aspects of good laboratory practices [1]. Current GCLP standards encompass applicable portions of 21 CFR parts 58 (GLP) [2] and OECD Principles of GLP [3]; and 42 CFR part 493 Clinical Laboratory Improvement Amendments (CLIA) [4].
By following the intent of all three regulations in combination, we can provide our clients with results and documentation that meet both preclinical IND sponsor requirements as well as clinical trial testing and reporting requirements.
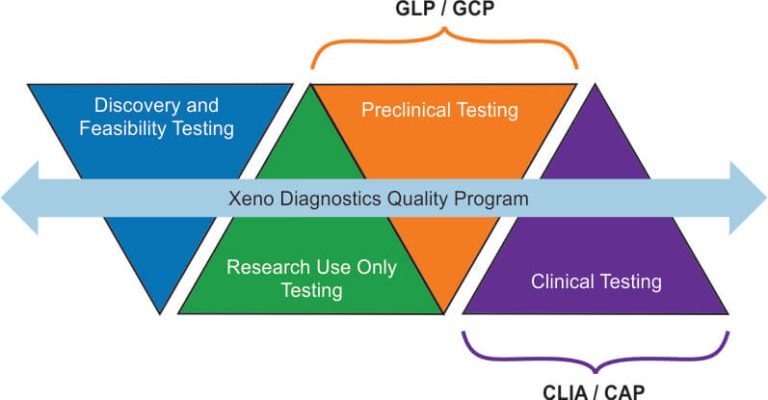
CLIA regulations apply to clinical testing. GLP regulations apply mainly to nonclinical testing conducted to support marketed products that are regulated by governmental agencies. GLP principles are generally applicable for animal safety or toxicology studies (however, the scope of testing has been gradually broadening over the years to include some clinical situations). The goal of GLP is to ensure the uniformity, quality and integrity of the results produced in these studies.
The goal of CLIA is to protect patients from testing that is done improperly which could cause harm to a patient. This would include inadvertently testing and reporting results on the wrong specimen, reporting results which were erroneous, reporting wrong test units, testing inappropriate specimens (inappropriate anti-coagulant used in collection, specimen collected at wrong time point, wrong patient tested, etc.)
Testing for CLIA generally starts with an FDA approved testing protocol that requires the laboratory to verify that it can perform the assay within the manufacturer’s standards. Should the test be a laboratory developed test (LDT), then the laboratory is required to perform testing for accuracy, precision, interferences, linearity, reportable ranges, analytical ranges, establishment of controls, participation in proficiency testing, types of specimens tested, specimen rejection criteria, and demonstrate testing personnel competency. These same parameters are required of all CLIA tests but for LDT, the support testing is much more rigorous.
In both GLP and CLIA testing, documentation should be such that all testing materials used to test a specimen or patient specimen can be identified. This is important in cases where patient results do not match the clinical picture and testing is called to question. Maintenance of the testing materials, controls, and verification of proper instrument performance at the time of testing will assist in validating the proper performance of the test. This is the same case for all GLP tested samples. The key in both systems is traceability and documentation.
Good Clinical Laboratory Practice (GCLP) provides guidance on utilizing Good Laboratory Practice (GLP) and Good Clinical Practice (GCP) principles when analyzing samples from a clinical trial [5]. At the same time, it ensures that the objectives of good clinical practice principles are achieved as well as the reliability and integrity of data generated [6]. By following the GCLP principles, Xeno Diagnostics can be trusted to provide clients with reliable, reproducible, and auditable results of the highest quality.
written by Daniel Wierda, PhD and Daniel Follas, MS
Copyright © 2021. All rights reserved.