Regenerative medicine is a discipline of medicine that focuses on the repair or replacement of damaged or diseased cells or tissues. Many institutions are developing novel methods to use naturally occurring cells, transgenic cells, derived cellular products, or engineered products to affect the repair or replacement process. Xeno Dx has been involved with testing umbilical derived CD34+ cells, MSCs, and novel biologically derived products throughout the discovery, development, and application in the regenerative process.
Mesenchymal stem cells (MSCs) are one type of cell that promote wound healing activity. MSCs are adult stem cells found within bone-marrow, adipose tissue, and other places in our body. Stem cells are a type of progenitor cell from which all other cells derive. As such, MSCs are able to differentiate into several cell types such as osteoblasts, chondrocytes, and myocytes, and can replace and repair damaged bone, cartilage, and muscle tissue respectively. One unique property of MSCs is the ability to be an immunomodulator by actively suppressing pro-inflammatory immune responses. This property has made the use of MSCs a very desirable avenue for regenerative medical therapies.
In regenerative medicine, Mixed Lymphocyte Reactions (MLR) can be used to assess how effective a treatment is at suppressing an unwanted inflammatory response (such as towards damaged tissue) and begin to promote wound repair and healing. By tailoring the readout method used for MLRs, we can observe the reduction in pro-inflammatory cellular signals and can even monitor the activity of cells that take part in the immunosuppressive and repair process.
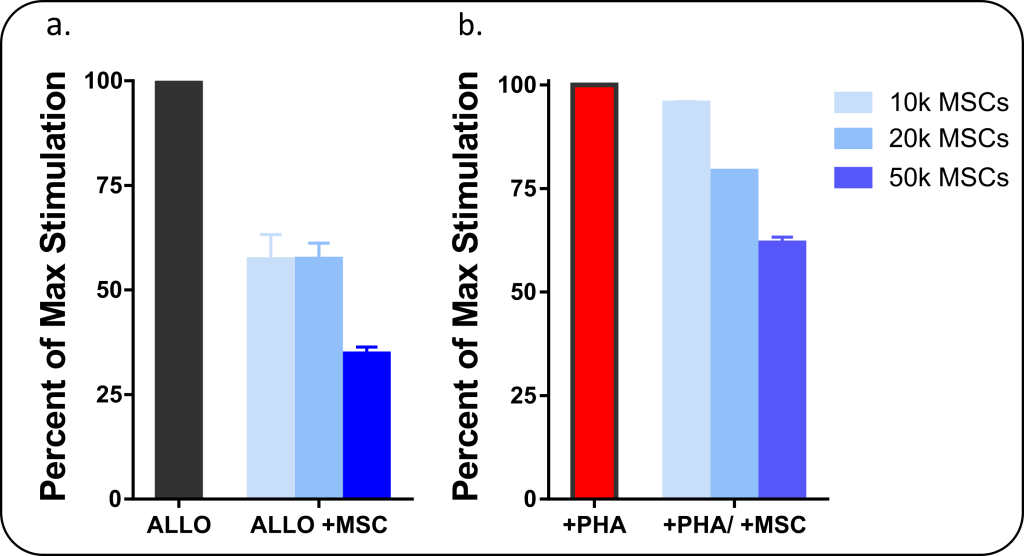
The figure below illustrates how regenerative medical therapies such as the use of MSCs can be analyzed for immunomodulatory properties using an MLR assay. In this assay, peripheral blood mononuclear cells (PBMCs) are stimulated to produce an inflammatory response (simulating an injury), and MSCs are added at different concentrations (10, 20, or 50k cells/well) to assess changes in T-cell proliferation. Figure 1a shows PBMCs being stimulated in a two-way MLR where two groups of PBMCs obtained from unrelated donors are mixed together to produce an Allogeneic (ALLO) stimulation response. Measuring cell proliferation of PBMCs caused by the ALLO stimulation, we see that MSCs have caused a substantial reduction in stimulatory response. Figure 1b, which uses a different stimulation method (phytohemagglutinin) to stimulate PBMCs, shows a similar response in MSC-induced decrease in cell proliferation as measured by flow cytometry. As the mechanism for MSCs inhibition of inflammation is mediated by the release of soluble cellular signals, it is also useful to investigate the anti- and pro-inflammatory cytokines released during the MLR. This can be analyzed by ELISA or multiplex (Luminex) analysis.
Figure 1: Mesenchymal Stem Cells (MSCs) have immunosuppressive activity when cultured with PBMCs immunogenically stimulated with a) allogeneic reaction (co-culture of two unrelated PBMC donors) with analysis performed by BrdU ELISA and b) phytohemagglutinin (PHA) a mitogenic stimulator of T-cells with analysis performed by Flow Cytometry.
We assist clients who are developing MSC based products through flow cytometric based phenotyping.
Please call or email if you have an idea for a custom project that utilizes our technical capacity or a similar idea/modification to the assays above. We are a fully equipped research lab with immunological technical expertise.
Technical Capacity/Technologies Utilized:
"*" indicates required fields
Copyright © 2021. All rights reserved.