Flow cytometry derives its name because the instrument flows the sample (cells) past a laser/detector. The sample flow stream is narrowed so that the cellular contents form a single file line where the narrow width prevents multiple cells from passing by the detector at the same time. Thus, flow cytometry has single cell resolution (e.g. flow cytometry analyzes each cell individually).
This technology is what makes flow cytometry so powerful – it can identify and differentiate between different cell types, including very rare populations. Beyond just cell types, flow cytometry also has the ability to detect differences between cells of the same type. For example, in an experiment looking at T-cell stimulation among PBMC populations, flow cytometry can both differentiate T-cells from other unwanted cell types (B-cells, monocytes, etc) and differentiate between varying degree of stimulation of the T-cells.
Flow cytometry accomplishes this by fluorescently tagging unique markers in or on the cell surface. These markers can be used to identify and determine cellular properties.
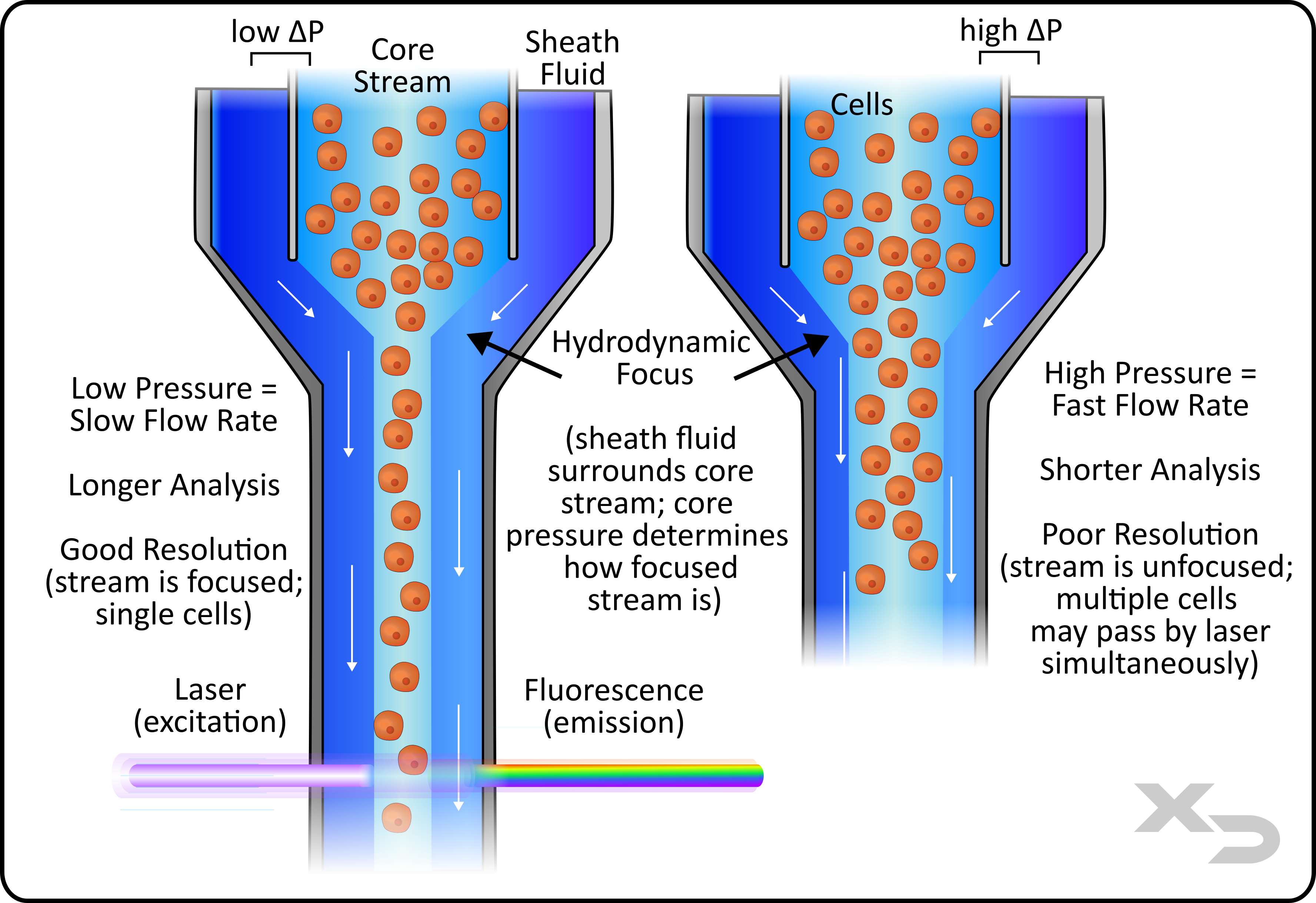
Figure: Simplified fluidics of a flow cytometer. Two variations are shown for low flow rate (left) and high flow rate (right)
Traditional flow cytometers dispense the sample (core stream) into the center of a buffer stream (sheath fluid). The sheath fluid forms a ring around the injected sample which compresses the sample core stream into a narrow stream. This is known as hydrodynamic focusing, and results in laminar flow of the core stream. The movement of the sheath fluid surrounding the core stream will drag/carry the core stream towards the interrogation point (e.g. laser/detector).
The compression of the sample (core stream) is important for signal resolution. Flow cytometers are intended to measure cells one at a time, collecting fluorescent signal for every individual cell. If multiple cells grouped together are allowed to pass by the laser/detector, then the resulting signal (co-incident events) will be useless for determining exact cellular properties and differentiating between types of cells.
For this reason the core stream needs to be narrowed until only one cell can pass by at a time. However, cells come in a variety of sizes, so the core stream diameter may need to be adjusted from study to study. This is done by altering the pressure difference between core stream and sheath fluid.
Doing so comes with an added change to the flow rate. If the pressure difference between core and sheath is increased, the velocity of the core steam does not change, but the width (cross-sectional area) does increase. This results in a higher volume of fluid moving past the interrogation point. High-pressure differences result in the opposite, where flow rates are faster at the cost of single cell resolution.
Other Types of Fluidics
There are several fluidic variations that are available in commercial cytometers. The two most common are acoustic focusing and microcapillaries.
Acoustic focusing uses sonic waves, in addition to hydrodynamic focusing, to further focus the cells into a single file beyond what hydrodynamic focusing alone can achieve.
Some instruments simplify fluidics by forgoing sheath fluid, and instead injecting samples into a microcapillary flow cell where the diameter of the capillary forces cells into single file streams.
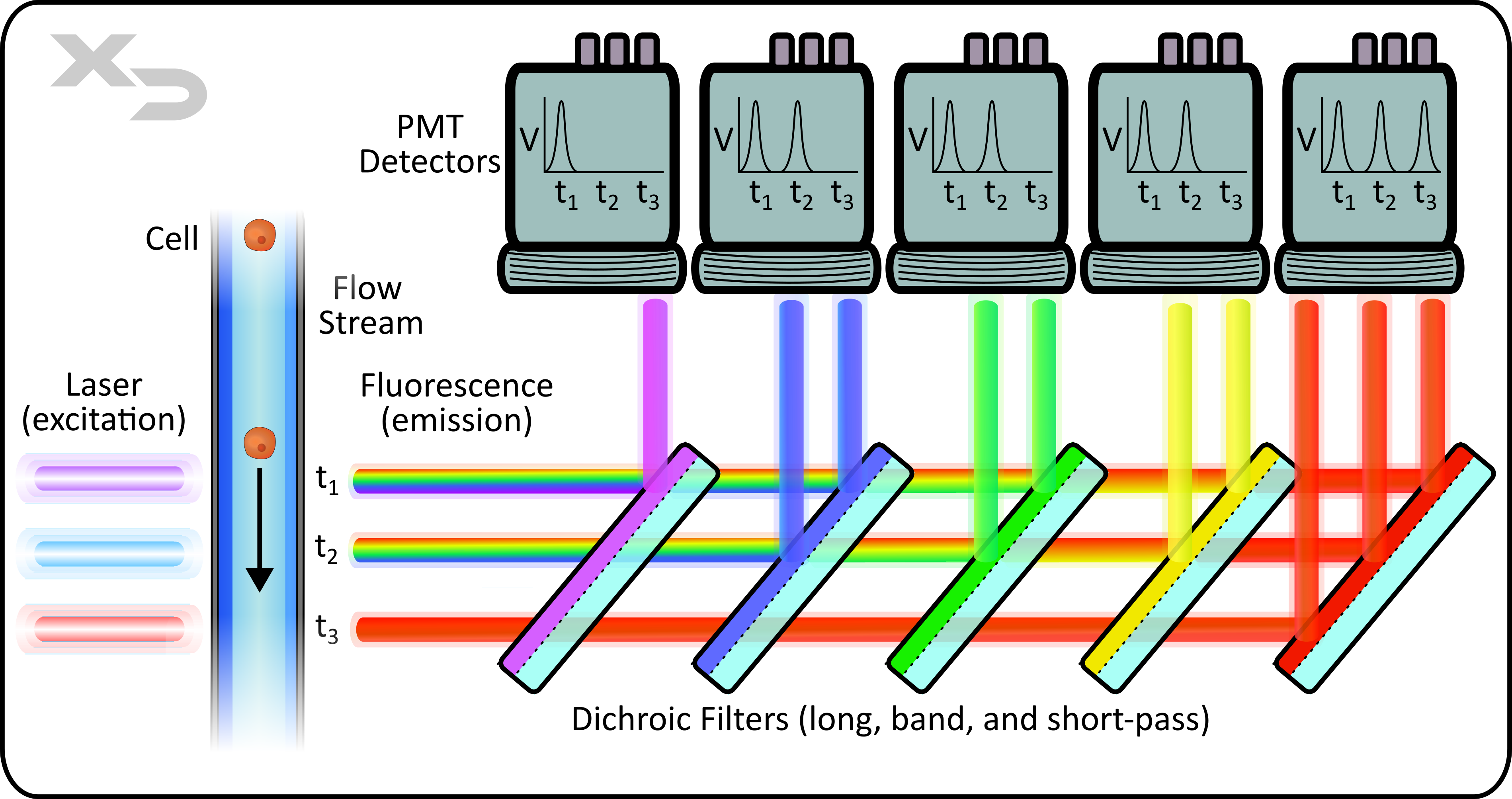
Figure: Simplified optics of a flow cytometer. Lasers are placed in a parallel formation with shared filters/detectors, where signal from a single cell is resolved in time for each laser/detector combination. Fluorescent emission from cell is reflected by a series of dichroic long and short band-pass filters. Photomultiplier tubes (PMT) detect emission photons and convert into voltage signal. Emission (light path and filters) are typically oriented at a 90° angle, perpendicular to laser path. Not shown: forward and side scatter detection.
There are three key components to the optics of a flow cytometer: Laser, Filter, and Detector.
Lasers
As the sample moves past the interrogation point, the laser illuminates the cellular sample and excites the fluorescent tags that are bound to the sample. For traditional flow cytometers, there are two types of laser setups: co-linear and parallel. In co-linear, the lasers are overlayed and interrogate the sample at the same time, whereas parallel lasers are physically separated and interrogate the sample at different locations (and different times). Co-linear laser instruments are limited in the number of fluorescent channels, as an individual detector will not be able to differentiate which laser caused a fluorescent signal; whereas parallel lasers can differentiate based on when the signal was received (resolved in time), or if each laser has its own unique set of filters and detectors. As such co-linear laser instruments are limited to 3-5 colors, whereas parallel laser instruments can achieve upwards of 10+ colors.
Filters
Typically, flow cytometers employ the use of dichroic filters, which are a type of band-pass filter. Dichroic filters will transmit (pass) specific wavelengths of light, and then reflect the rest. Each filter is paired with an individual detector, which detects the reflected light. This allows for discrete measurements of fluorescent signals spanning across the visual spectrum.
Detectors
The two most common types of detectors are photodiodes (PD) and photomultiplier tubes (PMT). In essence, both translate photon detection into a voltage output, however their mechanisms and uses differ. PD’s are simple inexpensive detectors that directly translate detection into a signal using a diode, consisting of a cathode/anode that excites when the anode is struck by photons. However, the resulting output voltage from a single photon is functionally undetectable, making PD’s a poor choice when trying to measure very dim fluorescent signals. This is why PMT’s are often preferred, as they amplify the signal from a single incident photon. The amount of amplification can be tuned by adjusting the voltage applied to the PMT’s.
Some instruments will have dedicated detectors for each fluorescent channel, whereas other instruments may save on costs, complexity, and foot-print size by sharing the same filters and detectors.
Conventional (Polychromatic) Flow Cytometry
Fluorescent Activated Cell Sorting (FACS)
Spectral Flow Cytometry
Cytometry by Time of Flight (CyTOF)
We have designed several informational pages with custom graphics to help explain the complex concepts of flow cytometry.
"*" indicates required fields
Copyright © 2021. All rights reserved.