Our CD34 enumeration is performed according to the guidelines laid out by the International Society for Hematotherapy and Graft Engineering (ISHAGE). Also included in our CD34 enumeration is a Nucleated RBC count, and adjustment. This test is required for AABB compliance.
In addition to CD34 enumeration, many clients request viability counts for their cord blood specimens. This testing offers more insight into processing efficiency. Our viability counts are performed as part of the CD34 enumeration, using 7AAD to differentiate live versus dead cells.
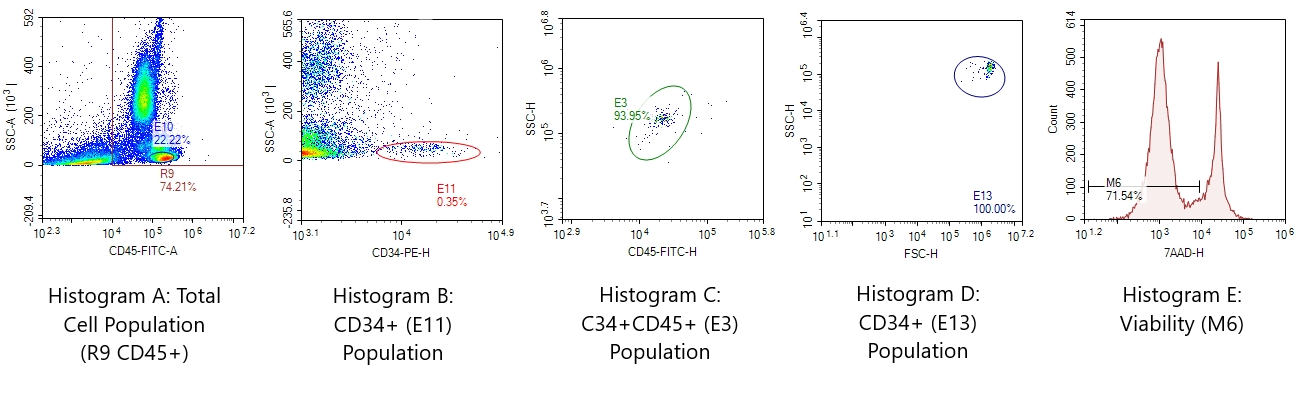
Figure 1 – Sample CD34 Flow Cytometry Results. Sequential (Boolean) gating techniques following ISHAGE guideline are used to define the CD34+ stem cells with the combination of viability assay, 7-amino actinomycin-D (7-AAD) dye. Nucleated cells are identified as CD45+ cells in Gate R9 (Histogram A), and CD34+ stem cells are identified as CD45+/CD34+ in Gate E11 (Histogram B). The cluster of CD34+ stem cells are serially analyzed regarding to CD45/SSC in Gate E3 (Histogram C) and to FSC/SSC in Gate E13 (Histogram D) to verify the cluster. The viability of nucleated cells (CD45+) is also measured (Histogram E) simultaneously.
Sample Type: Processed Human Cord Blood
Sample Volume: Minimum of 0.5 mL
Collection / Shipping Requirements: Do not centrifuge samples, Do not freeze
Turnaround-Time: 24 hours upon receipt
References
Sutherland DR, Anderson L, Keeney M, Nayar R, Chin-Yee I. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. International Society of Hematotherapy and Graft Engineering. J Hematother. 1996 Jun;5(3):213-26. doi: 10.1089/scd.1.1996.5.213. PMID: 8817388.
Copyright © 2021. All rights reserved.