There is no single “correct” way to perform an MLR (see MLR variations), rather there are many different readouts that can be utilized. Techniques that are used to assess cells, cellular contents, or cellular secretions can often be paired with the MLR. Corresponding readouts are tailored to the specific purposes of the assay – whether that be assessing immunogenicity via pro-inflammatory cytokine production, or investigating the mechanism of action via kinase activity.
The discussion below addresses a wide range of options for MLR readouts. The techniques are separated by commonality. However, it is important to remember that less commonly used techniques are still very useful. The MLR is heavily customizable, allowing for flexibility in its use; including adaptation to use uncommon readout techniques when needed. The provided list is not comprehensive as there are many more uncommon and experimental analytical techniques that could be leveraged in cell-based assays like the MLR.
Because there are so many variations of the MLR (format, cell source, cell treatments, article type, cell amounts, media choice, culture time, and readouts), each MLR’s experimental design is unique. When performing an MLR, regardless if the readout is common or uncommon, it is important to verify that the assay performs as intended, and to optimize it as necessary. Our scientific team has decades of combined experience doing exactly this. Our MLR services are tailored to each client’s needs, providing them with the exact information necessary to address their question or regulatory requirement. Our expertise allows us not only to provide these bespoke assays with minimal time invested in optimization, but our immunological and regulatory expertise helps clients interpret the data and navigate the regulatory landscape at each phase of product development.
With the exception of radioassays and multiplexed fluorescent microscopy, Xeno Dx offers all of the readouts listed below.
Cell Proliferation:
Cytoplasmic Proliferation Dyes-
Cell proliferation in response to cell-mediated immune reactions, typically T-cell proliferation, can be assessed by flow cytometry using cytoplasmic proliferation dyes. These dyes stain the intracellular contents of the cell, and as the cells divide, half of the contents (and therefore fluorescence) are passed to each daughter generation. This allows not only tracking of proliferation, but also of how many generations (subdivisions) the cells underwent.
Additionally, flow cytometry has the added benefit of using phenotypic markers to differentiate cell types (CD4 T-cells from CD8 T-cells, Tregs, etc.). Cells from different donors can also be identified if they were stained with different cytoplasmic dyes prior to culture.
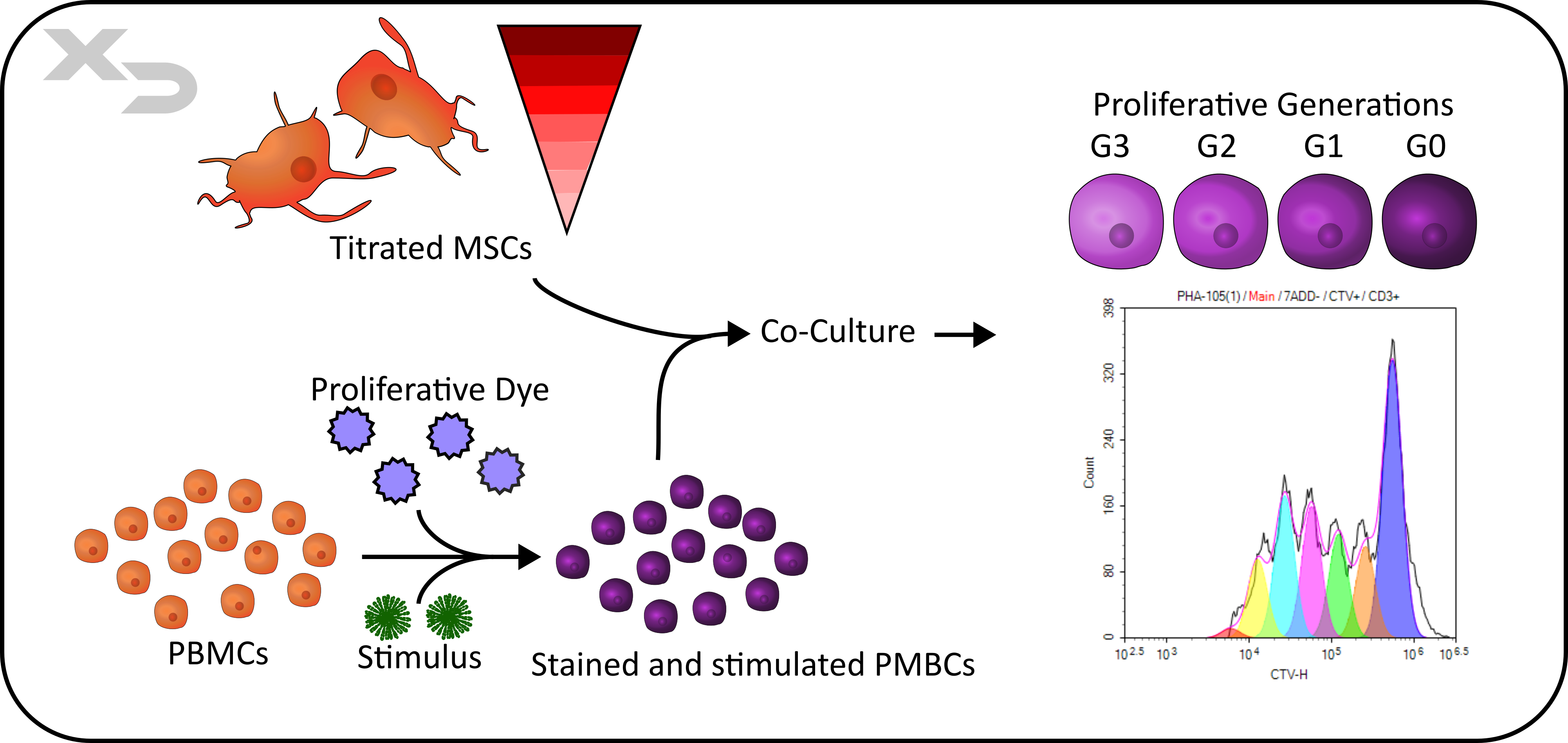
Figure: MSC-PBMC MLR Co-culture with cytoplasmic proliferation dye.
Thymidine Methods-
Another highly common method of assessing cellular activation and proliferation is by thymidine incorporation. Thymidine (and it’s RNA equivalent Uradine) is one of the four DNA nucleoside bases, and can be modified to allow for detection. Some common formats are the radioactive 3H-thymidine and the thymidine analogs: 5-ethynyl-2’-deoxyuridine (EdU) and 5-bromo-2’-deoxyuridine (BrdU).
3H-thymidine is detected via radioassay, where a scintillation counter records the radioactive beta-counts per minute; while the thymidine analogs are more flexible in how they are detected.
EdU functions as a click chemistry reagent (via copper catalyzed azide-alkyne cycloaddition; CuAAC), meaning that it forms rapid covalent bonds with high specificity (in this case specifically azides). Whereas BrdU can be detected with an anti-BrdU antibody. Both of these allow for flexibility, as either fluorescent labels or enzymatic tags can be attached to Edu’s azide conjugate or the anti-BrdU antibody for analysis (e.g. flow cytometry, ELISA, or other any other colorimetric and/or luminescent enzymatic assay).
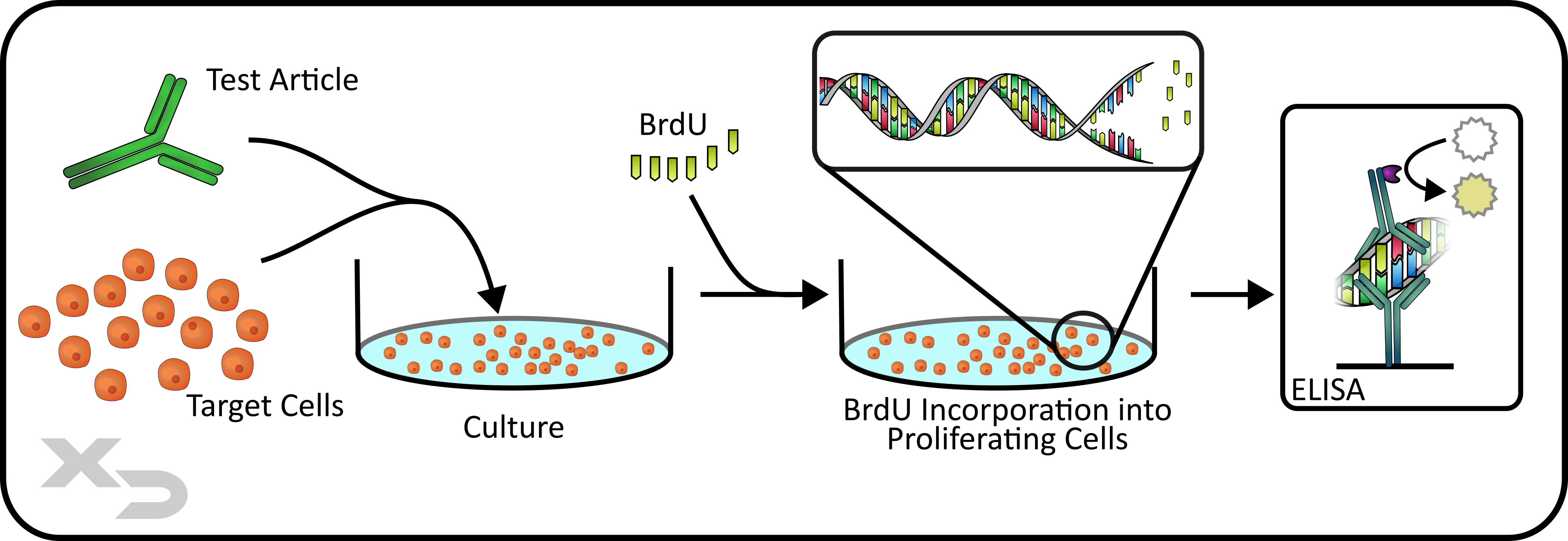
Figure: BrdU ELISA Proliferation Assay.
Other Techniques-
Other techniques for assessing cell proliferation are enumeration (e.g. counting by imaging or event counts in flow cytometry) and metabolic tetrazolium/formazan assays like XTT, MTT, WST-8, and others. While the tetrazolium/formazan assays are often simple to perform and give easy to analyze colorimetric data, they are limited in that the color production cannot be used to differentiate between donors or cell types. The amount of color production depends on the both cell viability and the number of cells. Thus, it is possible to misinterpret highly proliferative cells as non-proliferative if viability was lost over time due to toxicity or nutrient depletion (overgrowth).
T-Cell Activation Markers:
T-cell activation and proliferation is often accompanied with the expression of several common surface markers that can be used to identify activation: CD25, CD69, and CD71 among others. These are usually detected via flow cytometry. Depending on the stimulation conditions and mechanism, these markers will often upregulate and persist at different rates. For example, CD69 is an early marker that tends to dissipate after 3-4 days of stimulatory culture.
Intracellular Cytokines:
Another analytical flow cytometric method is the intracellular staining of cytokines. Monensin and Brefeldin A are two treatments that are often used to disrupt golgi function and protein transport, thus trapping proteins (e.g. cytokines) which build up in the cells.
Secreted Cytokines:
Cytokines and other immune signaling molecules can be detected from the cellular supernatant (e.g. media). These can be quantified using a number of immunoassays such as ELISAs, multiplexed cytokine bead arrays (e.g. Luminex®), or electrochemiluminescence ELISAs (e.g. MesoScale Discovery – MSD).
Kinase Activity:
Cellular activation is accompanied acutely by intracellular signaling cascades. While many of these targets can be assessed in different ways, by far the most common (by flow cytometry) is phosphorylation by kinases. The technique, known as phospho-flow, and when paired with cellular assays like the MLR, can provide functional data to help characterize the mechanism of action of a therapeutic.
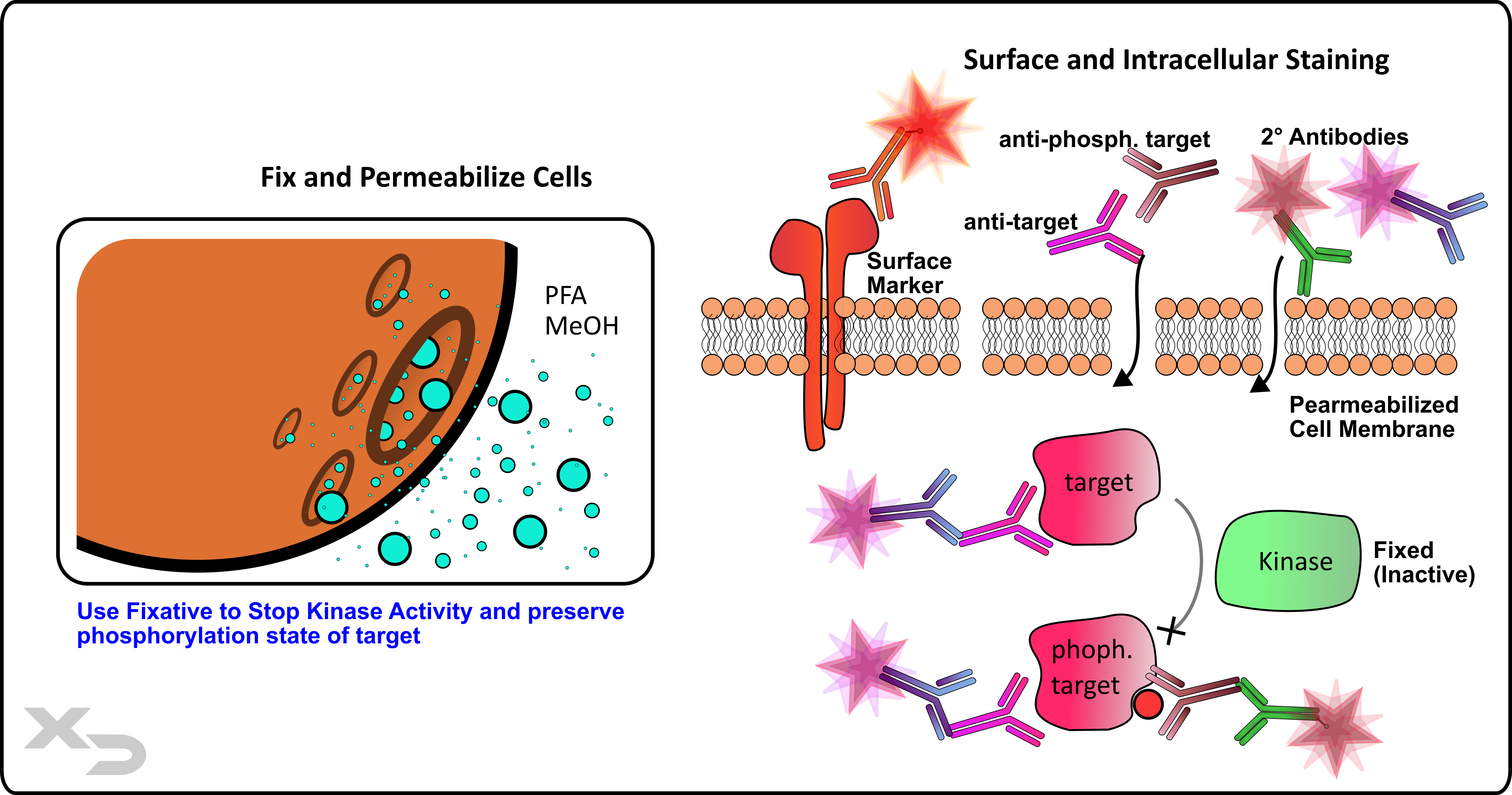
Figure: Phospho-flow analysis of kinase activity.
Phenotypic Changes:
Using flow cytometry, the phenotype of the cells in the MLR can be monitored for changes. This includes the reactive cells (PBMCs and T-cells) in the assay as well as cellular therapeutics (e.g. adoptive T-cells, CAR T-cells, MSCs, etc.). Cellular phenotypes are determined by expression of surface (or intracellular) markers (e.g. receptors) each with their own related function. As such, phenotypic changes can provide increased information about how the cells are reacting to a therapy (see activation markers for T-cell specific responses).
Viability and Apoptosis:
Viability of the cells in an MLR is a critical factor to assess, as it is important to determine if cellular response was not observed because the therapeutic was not immunogenic, or because the therapeutic was so cytotoxic that proliferation didn’t have a chance to occur. Viability can be assessed in a number of different ways, although it primarily is done via colorimetric dyes or fluorescent dyes. These can be assessed either via microscopy or flow cytometry, and there are a number of different dyes with different mechanisms (e.g. dead dyes vs live/functional dyes). Additionally, for assessing apoptotic cells, the protein Annexin V can be used to detect phosphatidyl serine (PS) that accumulates in the cellular membrane of apoptotic cells prior to death.
There are also a number of enzymatic assays to assess viability (or viability related measurements) such as tetrazolium/formazan assays (see proliferation), ATP assays (CellTiter-Glo®), LDH assays, Caspase assays, etc. Each can be formatted for use with the MLR.
Microscopy:
While flow cytometry is commonly used to assess cells obtained from MLR cultures, microscopy can also be utilized. Often times during culture, it is prudent to assess morphological changes in the culture by brightfield microscopy prior to end of culture and analyzed via another method. Alternatively, there are commercial instruments that allow for staining of cell cultures and long-term monitoring via fluorescent microscopic incubators.
Fluorescent microscopy is limited in the number of markers (colors) that it can use (3-5 colors) when compared to flow cytometry (10-20 colors); but still offers an attractive alternative depending on instrument availability or assay requirements (e.g. long-term monitoring).
Cell Cycle:
Cell phase (G0/G1, S phase, and G2/M phase) can be determined via the use of DNA dyes and thymidine analogs by flow cytometry. While not often employed in MLR assays, it can be used when the underlying mechanics of cell division and proliferation need assessed in greater detail.
ELISpot:
An alternative to traditional quantitative cytokine ELISA’s or multiplexed cytokine bead arrays is the assessment of cytokine secretion via ELISpot.
Cellular Lysates:
PAGE and Western Blots-
Cellular contents from MLR cultures can be collected, lysed, and assessed for specific intracellular or membrane components by polyacrylamide gel electrophoresis (PAGE) or western blot as needed. While flow cytometry is often the preferred technique to use due to multiplex capabilities and sensitivity, PAGE and western blot do have their uses. Electrophoretic separation allows for the determination of molecular weight, and western blot is an ideal medium for assessing cross-reactivity. Additionally, while reagent availability for flow cytometry is plentiful, there are occasions where uncommon antibody markers are only available for more traditional protein analysis applications like western blot.
mRNA Expression-
Analysis of mRNA expression by reverse transcriptase quantitative PCR (RT-qPCR) is often performed for more acute cell-based assays, where activation is measured in hours rather than days. However, RT-qPCR can still be used in the MLR when deemed appropriate.
Immunoassays-
Immunoassays (ELISA’s and Western Blots) can be performed on cellular lysates from the MLR if needed. The major advantage of ELISAs over flow cytometry and Western Blots is that they are quantitative, where at best Flow and WB can only be semiquantitative.
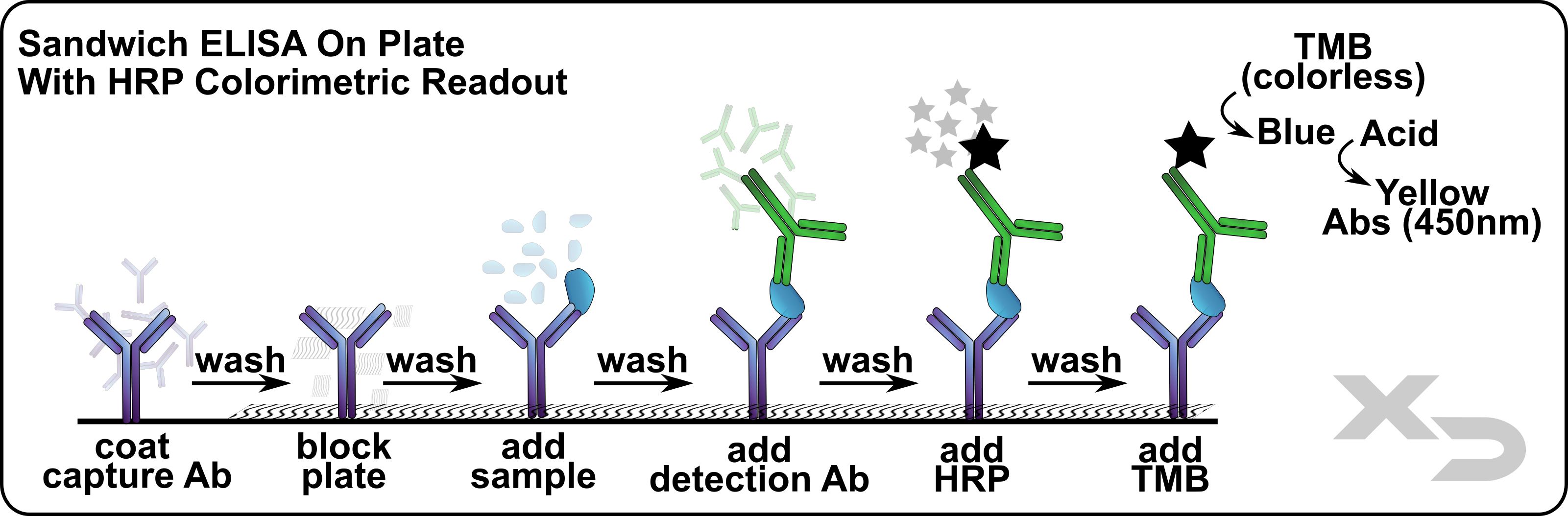
Figure: Sandwich ELISA schematic.
Enzymatic Assays:
As mentioned with proliferation and cell viability, there is a plethora of commercial assays that use enzymatic reactions to assess some feature or component of the cellular contents. Most if not all of these can, with some ingenuity, be adapted for use with the MLR.
It is worth stating again – the MLR is a culture technique and is not beholden to any specific analytical method. This gives the MLR a high degree of flexibility to fit most applications where cell-mediated immunological tests are needed.
How to select the right readout:
Selecting the right readout for your specific application can be a complex task, requiring careful consideration and advantages, disadvantages, and tradeoffs between reliability, ease of use, accuracy, and costs. That’s why Xeno Dx offers our expert MLR services. We tailor-fit every assay design and readout method to ensure that reliable and actionable results are obtained. If you would like to inquire more about our services click here.
MLRs model an adaptive immune response-
Want to know the immunological concepts behind the MLR? Checkout our webpage which discusses the minutia of cell-mediated immunity and how the MLR leverages those concepts to provide an informative assay.
Enhance your Development-
From evaluating mechanism of action to potency, and from immunogenicity to transplant tolerance, the MLR is a versatile platform that can be utilized for any therapeutics development pipeline. With many variations in format and readouts, the MLR can be custom tailored to fit your exact needs. Learn how the MLR can be leveraged to your benefit.
MLRs replicate our adaptive immune system via cell-cell mediated interactions. But just like our immune system, it is more complex than that. Cell interactions can be autologous (indirect allorecognition) or allogeneic (direct allorecognition). They can involve purified cells (e.g. DC/Tcell format) or they can use mixed populations (e.g. PBMCs). Cells can be from one donor or multiple, or different species. Each variation serves a different purpose and can be used to investigate different aspects of a therapeutic product’s immunological effects. Let us help you pick an MLR format that is right for you!
MLR Solutions For Industry Guidance-
MLR is a powerful tool for immunological research and can be a critical asset in many drug development pipelines. From Discovery to Pre-Clinical and Clinical Studies – no matter where you are in the process, Xeno Dx has an MLR service to help you!
"*" indicates required fields
Copyright © 2021. All rights reserved.