In-vitro assay for the evaluation of adaptive (cell-mediated) immune responses.
A Mixed Lymphocyte Reaction (MLR) is an in-vitro method to model an adaptive immune response to a drug or therapeutic product. In some cases, the MLR is also known as a Mixed Lymphocyte Culture (MLC), or for immunogenicity testing it may be referred to as PBMC assays, or CD8-T-cell depleted PBMC assays, or a DC/T-cell assay; all of which refer to the MLR in some format (see our page on MLR variations to learn how the MLR is a broader category of cell-based assays with different culture formats all revolving around the same principle mechanism of cell-mediated immunity).
An MLR replicates an adaptive immune response via cell-mediated immunity, where a mixture of lymphocytes (T-cells, B-cells, and NK cells) are cultured with antigen presenting cells (APCs). While there are several variations in how the assay can be designed, in essence, the MLR can be tuned to assess a wide variety of therapeutic articles, such as:
The MLR is one of the single best in-vitro tools available to assess how these therapeutics affect our immune system. It can be used to assess immunogenicity of the therapeutic, test potency and dose-response profiles, investigate mechanism of action, and characterize efficacy and bioactivity – whether it be immunosuppressive properties, checkpoint inhibition, or transplant tolerance. Each of these are critical factors to consider during product development and are often recommended tests for pre-IND applications or during pre-clinical and clinical studies.
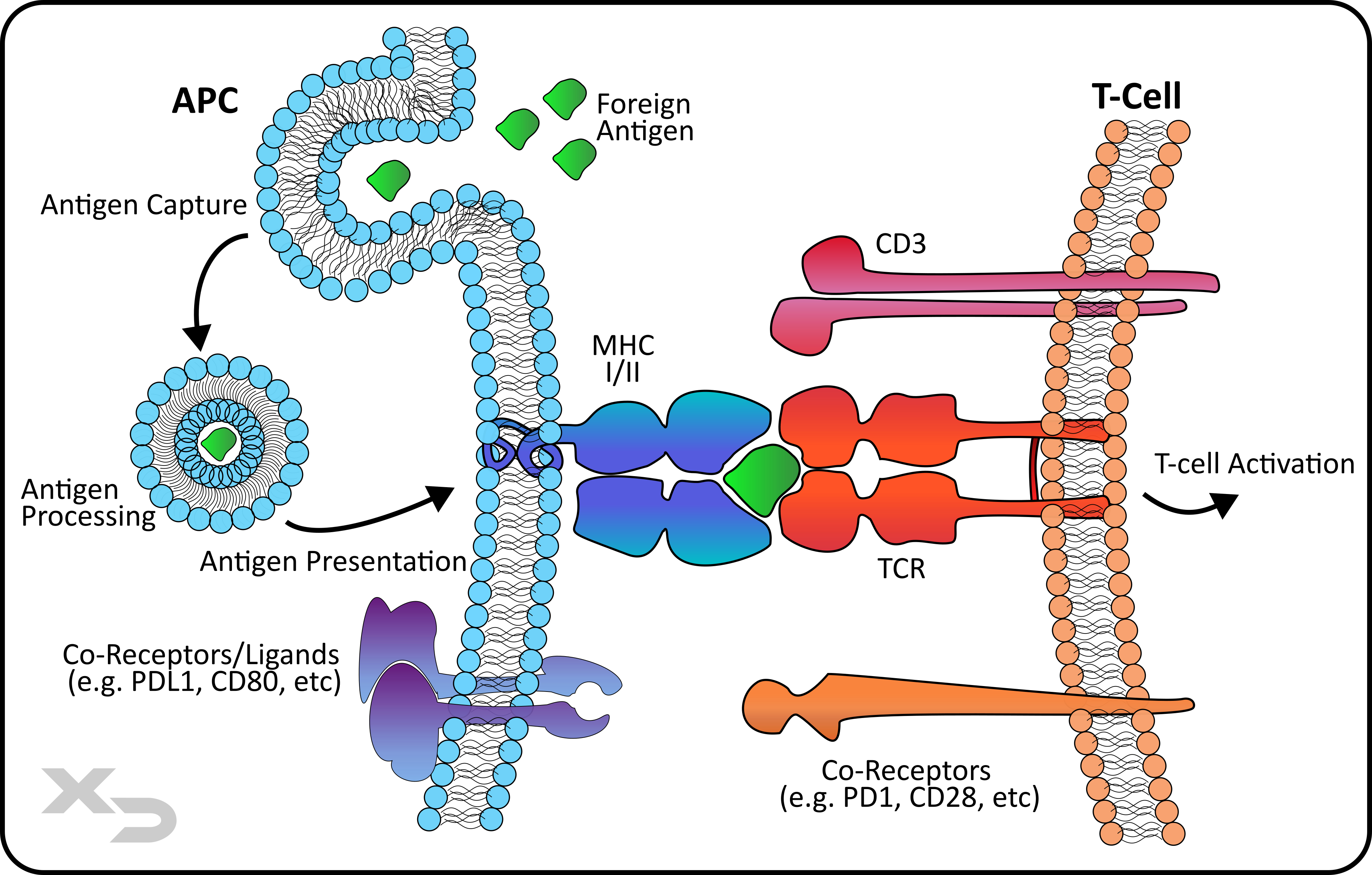
Figure: Simplified illustration of a cell-mediated signaling occurring between an Antigen Presenting Cell (APC) and a T-cell. The APC surface receptor – Major Histocompatibility Complex (MHC) presents the foreign antigen to the T-cell receptor (TCR). APCs come in two varieties: non-professional (MHC class I) and professional (MHC class II). MHC class II professional APCs also have a number of other co-stimulatory and checkpoint receptors/ligands can modulate the response. This is the key mechanism behind a mixed lymphocyte reaction (MLR).
Antigen Presenting Cells (APCs) and T-cells are at the heart of a cell-mediated adaptive immune response. APCs are a classification of immune cells that perform a similar function – to uptake, process, and present foreign antigens to T-cells. Antigen presenting cells activate T-cells by displaying a foreign antigen on a surface protein called the major histocompatibility complex (MHC).
APCs come in two varieties: non-professional (MHC class I) and professional (MHC class II). All nucleated cells display MHC I; therefore virtually all cells (except for red blood cells) can be antigen presenting cells. These are considered non-professional APCs as they 1) only stimulate CD8 cytotoxic T-cells, rather than CD4 T-helper cells (which coordinate the adaptive immune response); and 2) non-professional APCs lack co-stimulatory molecules (e.g. CD80) to fully stimulate T-cells. Professional APCs on the other hand are few in number: dendritic cells, macrophages, and B-cells.
T-cells are activated to either directly kill infected cells (CD8 cytotoxic T-cells) or to mediate the broader immune response by directing other cells to help fight the infection (CD4 helper T-cells). T-cell receptors (TCR) recognize and bind to the MHC-antigen complex, wherein the T-cells become activated and produce a number of downstream effects including: pro-inflammatory cytokine production, upregulation of T-cell activation markers (CD25, CD69, and CD71), as well as T-cell proliferation. This is the primary basis behind using the MLR to test therapeutics for immunogenic reactions, (e.g. to observe if the therapeutic produces an antigenic response via MHC-TCR cell mediated reaction).
There are also a number of co-receptors and ligands that can interact during antigen presentation (professional APCs) to enhance or modulate T-cell activation. These co-receptors, as well as other receptors and pathways are often ideal targets for immunomodulatory therapeutics. For example, immuno-oncology therapeutics may target checkpoint inhibitors like PD1/PDL1 to enhance T-cell reactivity, which is normally suppressed in the tumor-microenvironment. Other therapeutics may seek to suppress T-cell responses and an immune reaction, often for use with transplant patients.
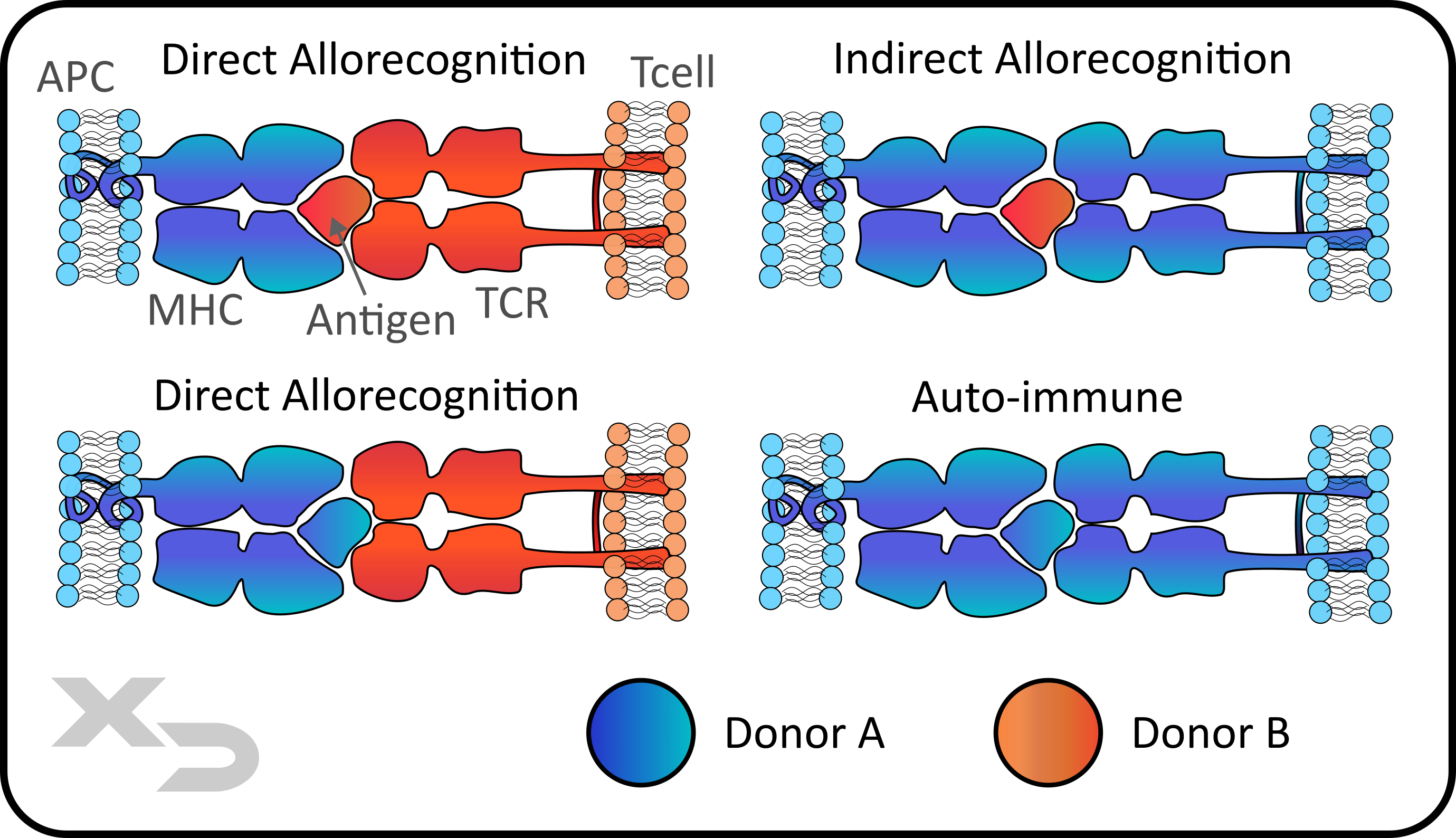
Figure: Methods of allorecognition (direct and indirect), as well as auto-immune.
Allorecognition is the ability of adaptive immune cells to differentiate between self and non-self (e.g. foreign cells or tissues). The MHC and TCR complex has several main ways in which it can react:
Whereas allorecognition is concerned with the mechanism of action (e.g. cell source AND antigen source), there is one other common way of categorizing/describing MLR reactions by cell source alone:
Enhance your development with a customized MLR
From evaluating mechanism of action to potency, and from immunogenicity to transplant tolerance, the MLR is a versatile platform that can be utilized for any therapeutics development pipeline. With many variations in format and readouts, the MLR can be customized to fit your exact needs. Learn how the MLR can be leveraged to your benefit.
MLRs replicate our adaptive immune system via cell-cell mediated interactions. But just like our immune system, it is more complicated than that. Cell interactions can be autologous (indirect allorecognition) or allogeneic (direct allorecognition). They can involve purified cells (e.g. DC/T-cell format) or they can use mixed populations (e.g. PBMCs). Cells can be from one donor or multiple, or different species. Each variation serves a different purpose and can be used to investigate different aspects of a therapeutic product’s immunological effects. Let us help you pick out which MLR is right for you!
Tailor MLR Readouts to Match your Needs
Decades of cell biology and immunological research have given us countless ways to measure and analyze a cellular response. At Xeno Diagnostics we use every technique at our fingertips. Absorbance, fluorescence, luminescence; ELISAs, Flow Cytometry, PCR, and more. Cells are complex and can respond in a myriad of different ways, so it only makes sense that we employ multiple strategies to assess as much as possible. Our Expert Team can pair multiple readouts for data-rich results, or hone in on the most critical factors with single readouts. Whether it is for lot release, potency, or mechanism of action, we can get you the results you need.
MLR Solutions For Product Development
MLRs are a powerful tool for immunological research and can be a critical asset in many drug development pipelines. From Discovery to Pre-Clinical and Clinical; no matter where you are in the process, Xeno Dx has an MLR service to help you!
"*" indicates required fields
Copyright © 2021. All rights reserved.